Hydroponics as the name suggests are the plants that grow in hydro(water) instead of soil. Traditionally, our farmers used to reap the crop and plough the field again to make it ready for the next harvest. So, ploughing the field was important right? Because that’s where the already used soil would go down and the nutrient-rich soil comes up on the surface.
So coming back to hydroponics, as every living thing needs nutrients for growth, hydroponics derive their growth formula from the nutrient solution which is often mixed with water and supplied to the plants in regular intervals. Basically, a hydroponic nutrient solution is a mixture of all the essential elements required for the healthy and sustainable growth of the plant and is also called a growth medium. There are 17 essential elements needed for most plants.
- 1. Components of hydroponics nutrient solution
- 2. pH of nutrient solution
- 3. How to Check pH Level of a Nutrient Solution
- 4. Maintain/adjust pH level of nutrient solution
- 5. Electrical Conductivity
- 6. EC to PPM/TDS Conversion
- 7. Temperature of nutrient solution
- 8. Commonly used Fertilizer for Hydroponic nutrient solution
1. Components of hydroponics nutrient solution
A nutrient solution is mainly made up of soluble salts of essential elements. The 17 essential hydroponic nutrient elements that are necessary for a plant’s growth and survival comprise carbon, hydrogen, oxygen, nitrogen, phosphorus, potassium, calcium, magnesium, sulfur, iron, copper, chlorine, and nickel which are categorized as macronutrients. Manganese, iron, zinc, boron, molybdenum are categorized as micronutrients.
Apart from carbon and oxygen as these are already available in the environment, all other nutrients are derived from the nutrient solution to prosper the growth of the plant. A simple type of hydroponic nutrient solution will mostly consist of nitrogen, phosphorus, potassium, calcium, magnesium, and sulfur, along with some micronutrients.
Here are some of the ideal nutrient solutions developed by researchers which are commonly used to manufacture the hydroponics solution. This table gives the data on how much quantity of each nutrient element is present in the Hoagland & Arnon solution, Hewitt solution, Cooper solution, and Steiner solution.
Note: All units in milligram per litre ( mg/L)
| Nutrient | Hoagland & Arnon (1938) | Hewitt (1966) | Cooper (1979) | Steiner (1984) |
| Nitrogen (N) | 210 | 168 | 200-236 | 168 |
| Phosphorus (P) | 31 | 41 | 60 | 31 |
| Potassium (K) | 234 | 156 | 300 | 273 |
| Calcium (Ca) | 160 | 160 | 170-185 | 180 |
| Magnesium (Mg) | 34 | 36 | 50 | 48 |
| Sulphur (S) | 64 | 48 | 68 | 336 |
| Iron (Fe) | 2.5 | 2.8 | 12 | 2-4 |
| Copper (Cu) | 0.02 | 0.064 | 0.1 | 0.02 |
| Zinc (Zn) | 0.05 | 0.065 | 0.1 | 0.11 |
| Manganese (Mn) | 0.5 | 0.54 | 2.0 | 0.62 |
| Boron (B) | 0.5 | 0.54 | 0.3 | 0.44 |
| Molybdenum (Mo) | 0.01 | 0.04 | 0.2 | – |
Hydroponic farming becomes a little technical as you have to take care of each and every parameter required for the growth at every stage of the plant life cycle. In traditional farming, we just leave it to nature to do most of the nutrition but it is very important to understand the nutrition solution if you want to get any success in hydroponic gardening.
Let’s talk about them one by one.
2. pH of nutrient solution
Understanding the concept of pH level in the nutrient solution is really important because the pH level of the solution will determine whether your plant absorbs the micro and macronutrients that you intend to give or not. Before jumping onto the mechanics of pH for hydroponic solutions let’s take a brief moment to understand pH in general.
pH or potential of Hydrogen is a measurement factor that denotes how much acidic or alkaline a mixture is. So, the amount of Hydrogen ions present in your solution is inversely related to the level of pH. More the number of H+ ions lesser will be the pH and the solution will be acidic and vice-e-versa. Water has a pH of 7 which is considered neutral.
So when it comes to the nutrient solution, maintaining the correct pH level of the solution is necessary otherwise your plant will not grow as desired. The optimum pH level for growth is said to be between 5.3 – 6.5. If the pH level of the solution falls below 5, the absorption of micronutrients like iron, manganese and copper becomes easy which creates scarcity of the macronutrients. If the pH level increases i.e the solution becomes alkaline then macronutrients dominate the absorption which leads to a shortage of micronutrients.
Here is a diagram to show the range of pH levels in which certain nutrients are more available than others. The thickness of each nutrient band is proportional to its availability.
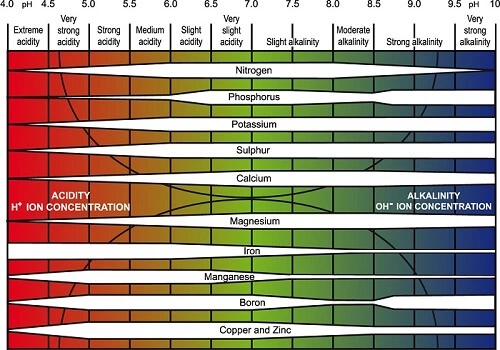
Fact: Potassium is completely available as a free ion in a solution having a pH in the range of 2-9. Iron, Copper, Zinc, and Manganese become unavailable at a pH higher than 6.5.
Now when we have understood the significance of pH, it becomes critical to know how to check and maintain the pH level of our solution so that your hydroponic system does not destroy the plant growth.
Let’s quickly glance at how you can check the pH level of your nutrient solution.
Also Read “How to Grow Hydroponic Lettuce At Home”
3. How to Check pH Level of a Nutrient Solution
There are three methods by which you can test the pH level of your hydroponic nutrient solution and those are using a litmus paper, using a pH-sensitive testing liquid, and the third being a pH testing meter.
You must have heard of something called a Litmus paper. Right? When we dip it into any liquid or a mixture it turns either blue or red where blue indicates alkaline and red indicates acidic. Well, you can use one of those pH testing litmus papers to gauge the pH level of your solution. It is the most simple and cheap way. The results are not too accurate.
With a pH-sensitive testing liquid, you can get a little better results and it costs a little extra to get one than a litmus paper. You have to take a sample out from your existing solution and then drop some of the testing liquid and as the colour of the sample changes, you can tally it with the scaling strip given in the test kit.
Using a pH testing meter is the best method out there. As it is a specialized equipment to test pH levels, the cost is a little on the higher side. The average cost of a pH meter on amazon.com is around $600.
For people who have large hydroponic systems or want to grow some complex plants and want to use their own custom made nutrient solution than having a pH testing meter is a must as it can save you from running your crops.
4. Maintain/adjust pH level of nutrient solution
For most of the ready-made nutrient solutions available in the market, the pH level is already mentioned but when you start to use the solution, as the plant absorbs the nutrients the pH level starts to change and hence it is absolutely essential to keep a check on your pH and maintain it regularly.
For most people, phosphoric acid and potassium hydroxide are the go-to solutions to increase or decrease the pH level respectively as both of them are readily available in the market and are inexpensive as well.
For the people who have just started with hydroponics are trying out their own nutrient solution for the very first time, ready-made pH maintainer solutions are also available which makes the job much easier.
5. Electrical Conductivity
The electrical conductivity of a hydroponic nutrient solution is a measure of the number of free ions that help the plants to grow. More is the electrical conductivity more is the concentration of the ions or nutrients. The concentration of nutrients needs to be maintained because if the solution becomes too concentrated it will create a hindrance to the flow of ions from the solution to the roots causing a nutrient deficiency.
For Example, in research, it was observed that if a rose crop is grown in a close hydroponic system, the concentration of iron decreases very fast as the crop absorbs all the available nutrients while the concentration of calcium, manganese, and chlorine starts to increase. Therefore, a balance needs to be maintained.
Measurement of EC is done with the help of an electrical conductivity meter and it gives the result in the number of ions present denoted by parts per million (ppm). The EC of each nutrient solution is mentioned on the label. So do check before you buy.
The best PPM for a hydroponic nutrient solution depends on the type of plant you want to grow. The electrical conductivity threshold for some of the common plants is given in the table below.
| Salinity Group | Threshold EC (dS/m) | PPM 500 | Plants |
| Sensitive | 1.4 | 700 | Lettuce, carrot, strawberry, onion |
| Moderately Sensitive | 3.0 | 1500 | Broccoli, cabbage, tomato, cucumber, radish, pepper |
| Moderately Tolerant | 6.0 | 3000 | Soybean, ryegrass |
| Tolerant | 10.0 | 5000 | Bermuda-grass, sugarbeet, cotton |
6. EC to PPM/TDS Conversion
In case you are wondering how to convert EC units into PPM units then let me walk you through it. The Electric Conductivity meter measures in EC units or dS/m but manufacturers use PPM to demonstrate the concentration of a nutrient solution. Also, different countries use different PPM scales, in the USA they use a 500 scale or a 650 scale and in the UK a 700 scale is used.
So this is how you convert EC to PPM
In USA
PPM500 = EC x 500 or,
PPM650 = EC x 650
In UK
PPM700 = EC x 700
7. Temperature of nutrient solution
Research has shown that the temperature of the solution plays a critical role in the oxygen absorption pattern and absorption of nutrients by the plants. The temperature mainly affects the uptake of water by the roots. In indoor systems for example a home setup or a greenhouse, the temperature is maintained naturally with the help of cold water in summers and a water heater in the winters.
It becomes difficult to regulate the temperature for an outdoor setup directly in contact with the natural atmosphere.
When two rose plants were kept at two different temperatures 50 F and 70 F, it was observed that the intake of NO3– increased and water intake decreased in the one with lower solution temperature. The quantum yield was also higher in the one with a 50 F solution. For spinach, it is seen that the optimum level of temperature to obtain potential yield is 82.4 F.
8. Commonly used Fertilizer for Hydroponic nutrient solution
The table given below sums up the commonly used fertilizers and acids used in hydroponic agriculture. I hope this will help you get an idea of what fertilizer to use with your nutrient solution.
| Fertilizers | Formula | Nutrient Percentage | Solubility (g/L at 68 F) |
| Calcium NItrate | Ca(NO3)2. 5H2O | N:15.5, Ca: 19 | 1290 |
| Potassium Nitrate | KNO3 | N: 13, K:38 | 316 |
| Magnesium Nitrate | Mg(NO3)2. 6 H2O | N:11, Mg: 9 | 760 |
| Ammonium Nitrate | NH4NO3 | N: 35 | 1920 |
| Monopotassium Phosphate | KH2PO4 | P:23, K:28 | 226 |
| Monoammonium Phosphate | NH4H2 PO4 | N:12, P:60 | 365 |
| Potassium Sulphate | K2SO4 | K:45, S:18 | 111 |
| Magnesium Sulphate | Mg SO4. 7 H2O | Mg:10, S:13 | 335 |
| Ammonium Sulphate | (NH4)2 SO4 | N:21, S:24 | 754 |
| Potassium Chloride | KCl | K:60, Cl:48 | 330 |

